Mass Eye and Ear otolaryngologists Christopher Hartnick, MD, and Phillip Huyett, MD, are pushing the envelope in sleep apnea treatment using hypoglossal nerve stimulation. Watch this video of Drs. Hartnick and Huyett describing their work at the 2022 Sense-ation! Gala, and hear one family’s story.
Updated March 21, 2023: The U.S. Food and Drug Administration approved the Inspire device for adolescents with Down syndrome and obstructive sleep apnea ages 13 and up.
The consequences of obstructive sleep apnea go beyond just snoring and waking up in the middle of the night. People with the condition regularly experience short-term challenges at work or school caused by poor sleep. They are also at greater risks of acute health problems, such as stroke, heart attacks and dementia, in the future.
While many people diagnosed with obstructive sleep apnea benefit from conventional therapies, like continuous positive airway pressure (CPAP) therapy, not everyone can tolerate the treatment. This is especially true for a segment of the population at a significantly greater risk of obstructive sleep apnea: children with Down syndrome.
Two Mass Eye and Ear surgeons are pioneering how a device called a hypoglossal nerve stimulator can be a game-changing treatment for those patients.
Newer option for adults with sleep apnea
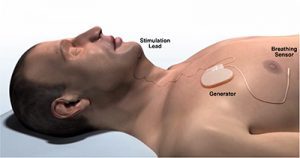
In 2014, the U.S. Food and Drug Administration (FDA) approved the Inspire Upper Airway Stimulation (Inspire Medical Systems©) hypoglossal nerve stimulator, a battery-powered implant that offered adults a new treatment option for sleep apnea. The device is surgically placed under the skin above the chest and is connected to the hypoglossal nerve, which controls tongue movement. The device is turned on before bed with a remote control and, during the night, acts as a “pacemaker for the tongue” by moving the tongue slightly forward with each breath, relieving the obstruction in the airway causing sleep apnea.
Phillip Huyett, MD, director of sleep surgery, is one of the country’s leaders in using this procedure to treat adults with obstructive sleep apnea, performing more than 300 implantations of the device since launching his program at Mass Eye and Ear in 2019. Through his expertise and experience, Dr. Huyett has been able to modify the procedure and reduce the duration of the operation significantly.
“By helping a lot of patients with this therapy, we’ve made the procedure more efficient and have been able to get average OR (operating room) times from about two to four hours down to under 60 minutes,” he said.

Only three incisions are made during the surgery and patients go home the same day. After allowing the incisions to heal for up to one month, Dr. Huyett turns the device on to a minimal setting and teaches the patient how to use the remote.
More than 80 percent of patients at Mass Eye and Ear who have undergone this surgery have responded positively to the new device, according to Dr. Huyett. He is conducting ongoing research to track patients’ outcomes.
“It’s not uncommon for adult patients and their bed partners to call this a life-changing therapy,” Dr. Huyett noted.
Treating an underserved group: Children with Down syndrome
Children with Down syndrome are disproportionately impacted by obstructive sleep apnea: about 80 percent of kids with Down syndrome have the condition, compared to only 5 percent of the general pediatric population.
This is due in part to children with Down syndrome having a larger tongue and lower muscle tone that further restrict the airway, explained Christopher Hartnick, MD, director of pediatric otolaryngology at Mass Eye and Ear. What’s more, sensitivity challenges make wearing a CPAP mask extremely difficult.
“We would reach a point where we simply ran out of good options to treat apnea in these patients, which was frustrating for their families,” he said.
Affected families noted that their children not only had a poor night’s sleep, but the effects carried over to challenges with their behavior, difficulties at school, and issues with their spoken language.
After reading some research on hypoglossal nerve stimulation and its success in adults, Dr. Hartnick organized a clinical trial with his colleague Brian Skotko, MD, MPP, the Emma Campbell Endowed Chair on Down Syndrome at Massachusetts General Hospital, to test the procedure’s safety and effectiveness in adolescents with Down syndrome. The research team recruited 42 adolescents with Down syndrome and severe obstructive sleep apnea between the ages of 10 and 22; all underwent surgery to implant the Inspire hypoglossal nerve stimulator and were tracked for one year after.
In a study published in April, Dr. Hartnick and his team reported that 27 of the patients (66 percent) responded well to the therapy, with the number of apnea events per hour decreasing by at least 50 percent. On quality-of-life surveys, families reported their children had significant improvements in their daily functioning and behavior.
“These children were no longer the first ones sleeping through their classes, nor were they the first ones heading to bed at a sleepover,” Dr. Hartnick said. “This is the next step forward in allowing them to participate in school, transition from childhood into adulthood and hold jobs.”
A “magical” change in Elliot
Kate Dougherty watched her son Elliot struggle with obstructive sleep apnea his entire life. While other kids could stay up late at sleepovers, Elliot — who has Down syndrome —was often the first one asleep. He needed daytime naps and would have challenges at school and problems with focusing.
Kate, who recently became president of the National Down Syndrome Congress, had spent all of Elliot’s life advocating for him and searching for the best care and resources possible. Her research led her to Dr. Hartnick’s clinical trial, which she enrolled Elliot in when he was 10 years old. Despite living across the country in Taylor, Missouri, Kate and Elliot and the rest of their family would make frequent trips to Boston for surgery and follow-up visits. Now 13, Elliot’s mom says the differences she sees in her son are clear as day.

“It was magical,” Kate said. “He can stay up late with his friends and have overnights. He’s the last man standing. His school is beyond thrilled because he has a clarity of speech that was unknown before. His other doctors are thrilled because we can get reads on his speech and language, and all of which have shown incredible improvement in the three years since he’s had the implant.”
Kate added, “It’s unbelievable. It’s a miracle.”
Buoyed by the feedback from parents, Dr. Hartnick and Dr. Skotko applied for and received a $4 million, five-year grant from the National Institutes of Health (NIH) to study if hypoglossal nerve stimulation might improve neurocognition and language in young patients with Down syndrome.
In March 2023, the FDA approved the device for treating adolescents with Down syndrome over 13 years of age, thanks in part to the research led by Dr. Hartnick. Even with this approval, the team will continue this research and is still recruiting patients, as it may contribute to lowering the indicated age for beginning treatment, according to Dr. Hartnick.
“The Down’s syndrome community is very excited about this research,” said Dr. Hartnick. “And if this affords them a safe and effective option, then this is a real opportunity for families.”
Read more about Dr. Hartnick’s trial from STAT and NBC News.



Do you treat central sleep apnea
I also have OSA
I have Inspire which has only reduced my AHI by 17% and a Herbst appliance by 37% while using the Inspire
I’ve lost the challenge of wearing a Bipap
Do you utilize the REMEDE procedure to treat central sleep apnea
Hi Eric, thanks for reading. It might be best to contact the Sleep Medicine and Surgery Division directly at 617-573-3954 to address your specific questions on treatment options.