3D-printed graft developed by researchers at Harvard’s Wyss Institute, Harvard SEAS, and Mass Eye and Ear to be commercialized by Desktop Health, following the startup’s acquisition
Advanced through a six-year effort in Harvard University’s translational research environment, a biomimetic hearing-restoration technology invented by a multidisciplinary research team at Harvard’s Wyss Institute for Biologically Inspired Engineering, Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and Massachusetts Eye and Ear, has taken an important step closer to patients.
The PhonoGraft device is a 3D-printed, biocompatible graft that could be implanted to repair a damaged eardrum. If its clinical development is successful, the PhonoGraft technology could mitigate the pain, drainage, and hearing loss associated with ear drum perforations that affect millions of individuals worldwide.
The technology is now entering commercial development. Driven to make their innovations available to patients, entrepreneurial members of the research team launched a startup company, Beacon Bio, with an exclusive license from Harvard’s Office of Technology Development (OTD) to commercialize the PhonoGraft innovations co-owned by Harvard and Mass General Brigham. Beacon Bio was acquired this summer by California-based Desktop Health, a healthcare business within Desktop Metal Inc. that focuses on new 3D printing and biofabrication solutions for personalized medicine. Nicole Black, PhD ’20, who was CEO of Beacon Bio, will continue to lead the platform as Vice President of Biomaterials and Innovation at Desktop Health.
“I am absolutely thrilled to have Beacon Bio become a part of the Desktop Health team, and to see these innovations advance so far from their earliest days in the lab,” said Dr. Black, who is a co-inventor of the PhonoGraft technology and spearheaded its development at Harvard and Mass Eye and Ear. “The general support, manufacturing expertise, and regulatory expertise of an established company will be key to bringing the PhonoGraft platform to patients and to developing a regenerative medicine platform around the technology.”

Dr. Black was initially a doctoral student at SEAS, studying materials science and bioengineering in the lab of Jennifer A. Lewis, ScD, the Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS and a Core Faculty member at the Wyss Institute. In developing the technology, the Lewis Lab teamed up with two surgeons at Mass Eye and Ear: Aaron Remenschneider, MD, MPH, and Elliott Kozin, MD. Going forward, Drs. Lewis, Remenschneider, and Kozin will participate in the scientific advisory board of Desktop Health.
The researchers at Harvard and Mass Eye and Ear designed the PhonoGraft technology to utilize a novel biomaterial-based approach that guides the regeneration of native eardrum tissue. Its 3D-printed structure mimics the structure of a normal eardrum and effectively stimulates the tissue’s self-healing properties, as demonstrated in animal models. The team believes the PhonoGraft material technology – unlike other methods – could potentially enable permanent repair by first closely mimicking and then restoring the eardrum’s sound-conducting mechanical properties and barrier functions. The eardrum, or tympanic membrane, is a thin membrane evolved to conduct sound in the ear and serves as a protective barrier against invading pathogens. The eardrum can become perforated by blasts, traumatic injuries, and chronic ear infections. Despite the eardrum’s remarkable self-healing powers, many perforations cannot heal by themselves and require reconstructive “tympanoplasty” procedures in which ear surgeons repair the hole with tissue grafts harvested from the patient. Even with modern techniques, surgical failures are common, making revision surgeries necessary. Additionally, patient-derived tissue grafts have imperfect sound-conducting abilities, as their structure does not match that of the normal eardrum.
Envisioning PhonoGraft technology as a regenerative medicine solution
“As ear surgeons, we commonly see patients with perforated eardrums from trauma, chronic infections, or blast injury who in many cases need surgical interventions, for which there definitely is room for improvement,” said Dr. Remenschneider, an otologist and neurotologist, researcher at Mass Eye and Ear, and lecturer at Harvard Medical School. “In the wake of the Boston Marathon bombing on April 15, 2013, many of the eardrum perforations were cared for at Mass Eye and Ear. It was really a catalyzing event that prompted us to look more systematically at our surgical techniques, grafting material, and outcomes, which eventually made us focus on improved eardrum grafting materials to promote better healing and hearing outcomes after eardrum repair.”
“Ear surgery has seen a lot of advances over the last 10 years. An important one has been the development of endoscopic procedures that allow us to treat patients directly through the ear canal, which in many cases avoids skin incisions and drilling behind the ear,” said Dr. Kozin, who is an otologist and neurologist at Mass Eye and Ear, and an assistant professor at Harvard Medical School. ”This approach, together with innovations from the Lewis lab, enabled us to imagine, design, and ultimately, manufacture a device that may one day improve the outcomes of eardrum surgeries.”
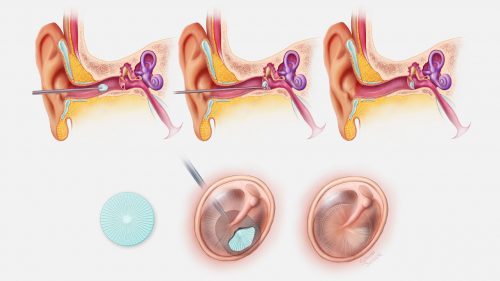
In 2014, Drs. Remenschneider and Kozin read an article in The New Yorker titled “Fugitive Ink,” featuring Dr. Lewis and her lab’s 3D printing feats. Soon after, they visited the Lewis Lab to give a chalk talk and explore whether her novel 3D printing method could be used to improve ear surgery. Following a discussion around outcomes after an ear drum surgery and the lack of suitable grafting materials, the newly formed team of surgeons and engineers set out to create a better functioning, next-generation graft.
“When I learned that the native tympanic membrane is composed of a web-like radial and concentric architecture, I was really excited, as it builds upon earlier work from our lab, in which we first demonstrated printing of 3D spider web-like structures,” said Dr. Lewis. Dr. Black, who had just joined the lab as a graduate student, embraced this idea because her goal was to create a new biomedical device to address patient needs.
“Despite this clearly defined problem, the challenges were numerous: we envisioned a device that could enable easier implantation, guide remaining eardrum tissue to remodel and repair the perforation, match the circular and radial structure of the eardrum to allow for efficient conduction of sound, and biodegrade to reduce the risk of biofilm formation that can otherwise result on permanent devices,” said Dr. Black.
A fruitful collaboration between Drs. Black and Lewis working at the Wyss Institute and SEAS, alongside Drs. Remenschneider and Kozin at Mass Eye and Ear, started to take shape with that clear and ambitious goal in mind: an implantable device to enable full reconstruction of the native eardrum in vivo, for which the team later coined the name “PhonoGraft.”
Read more from the Wyss Institute on the technology and new partnership with industry.

Would this device be availale for adults whosuffer from otosclerosis?
Hi Debbi, thanks for reading and your comment. This device is being looked at for perforated eardrums at this time.
This is amazing, and I look forward to being one of the first candidates to receive this application so that my perforated right eardrum can once again conduct sound as it did in my youth.
Thanks for reading and your comment Tomothy, we’re pulling for you!
Hello, I was just reading this article and very interested in your thoughts. At age 15, I came to MEEI EW for and earache in my left ear. My mother was in in the exam room as it was just an earache. The female tech or nurse? that saw me prior to the doctor coming in used a small metal thin tube to look into my right ear to see if there was wax buildup. I informed her that this is not the ear I have pain in, it’s my left eye. She said she had to check both. When she inserted the tube in my right ear, I had sudden severe pain and was told that it’s normal after an exam. A few min later, the doctor came in and looked into my left ear and then my right and said “holy sh** what happened?” I told him I don’t know but when the woman put the tube in my ear, I had excruciating pain. He left the exam room and came right back w/another doctor and told me that she punctured my eardrum and I had to have surgery ASAP. I was admitted into MEEI the next day , had the right side of my head shaved, and the hole was patched with skin from my right arm. and put some kind of gauze all the way in my ear and wrapped my head like a mummy. I was admitted for about 7 days. The recovery was brutal. and my summer was an anxious one with oozing, pain, itching and follow-up apts. I have been about 80% deaf in that ear since then. Not sure if I would be eligible for this trial or not.
Thank you
Corinne Powers
Hi Corinne, thanks for reading and your comment. We are sorry to hear about your experience years ago. We’ll continue to post updates on our trial and eligibility criteria on our blog as more information comes out.
Interested in finding out more as I have “flabby” ear drums, that are scared and thinning due to many year of tubes growing up as a child. I am told that hearing aids will not help my condition, outside of tightening up the ear drum or repairing damaged areas.
Hi Matthew, we’ll continue to post updates on our blog about this work. IF you’re interested in a consultation in general for your issue with a Mass Eye and Ear ear specialist, you can contact 617-573-3954. Here’s more information: https://www.masseyeandear.org/specialties/hearing-and-balance
Hi i need en appointmet vit dr ears i dont hear good i live i Stockholm sweden
Hi Russel, thanks for your message. International patients interested in medical care at Mass Eye and Ear can contact international@meei.harvard.edu. Here’s more info: https://masseyeandear.org/patients-visitors/international
I had ear implant at Mass Eye and Ear Hosiptal year 2012 They did Tympanoplasty first April 2012 3 months later install ear implant left ear everything was a sucess. Surgery by Ronald K.de Venecia Great job. I was 68 years old that time. Now I am 77 years old with perforation in right ear and lite infection. & no earing. Will this help me ?
Hi Robert, thanks for reading and leaving a comment. Glad to hear you had a great experience here at Mass Eye and Ear! We can’t provide medical advice on specific cases over the blog unfortunately, but we’ll post an update when there are clinical trial opportunities so our patients can learn the criteria to enter.
Very interesting to say the least! If eligible, I would be interested in participating in the clinical trials.
Thanks for reading and your comment, Amy. We’ll post an update on our blog when there are clinical trial opportunities.
In the ninties, while I was still smoking, I had the need to have my eardrums perforated to enhance my hearing. There was considerable fluid build up in the, eustacian canal, which was relieved after the procedure. Since quiting smoking in 2003 I have not had the problem. However, recently I have had some chronic earaches that has me wondering if they have any relation to those procedures.
Thanks
Tom Whalen
Hi Tom, thanks for reading and your comment. Those questions are best answered by your doctor. If you’d like to see a Mass Eye and Ear ear specialist, you can contact 617-573-3954. Here’s more information: https://www.masseyeandear.org/specialties/hearing-and-balance
This is exciting news. Congratulations! I have no left ear drum and would be interested in learning more.
Best
Gary
This is amazing. I had perforation for almost a year now. When phonograft will be available to patients? Have any clinical trials been done with it? what are the hearing outcomes?
Is this available for large perforated ear trum? If so, Product size is large enough to cover large perforation?
What happened to this technology and why isn’t it being talked about anymore?