Mass Eye and Ear glaucoma specialist Nazlee Zebardast, MD, MSc is harnessing the power of big data and artificial intelligence (AI) in an effort to provide more individualized glaucoma screening and treatment for patients.
Glaucoma is a leading cause of irreversible vision loss, yet some people are able to live for years without their vision being affected. Currently, there is no way to predict whose disease will worsen and whose will not.
Researchers at Mass Eye and Ear are looking to fill that crucial knowledge gap. A unique project led by Nazlee Zebardast, MD, MSc, a glaucoma specialist and researcher and Director of Glaucoma Imaging at Mass Eye and Ear, is utilizing AI to develop a personalized risk score that can help determine how to best care for a patient with glaucoma. This research may lead to better knowledge of how often a patient with glaucoma needs to visit their eye doctor, and also how aggressively they should be treated.
“We’ve known for a long time that not everyone with glaucoma is affected the same way. Some people may never lose vision, while others lose their vision rapidly,” Dr. Zebardast told Focus. “Our goal is to use what we have learned about glaucoma genetics and advanced imaging to offer more personalized glaucoma care for patients.”
Glaucoma’s “silent” impact on vision
Glaucoma is a group of disorders that damage the eye’s optic nerve, resulting in vision loss or blindness. In many cases, the nerve damage is caused by increased pressure inside the eye, but this disease can also occur at “normal” eye pressures. Glaucoma is often referred to as the “silent thief of sight” because this progressive nerve damage causes vision loss over time, often without a person realizing it. In fact, 50 percent of those with glaucoma in the U.S. don’t even know they have the disease.
“Because glaucoma is silent, we need to screen for it,” said Dr. Zebardast. “However, current screening methods are imprecise.”
Currently, measuring the pressure inside the eye is a major determinant of how doctors decide to best monitor and treat glaucoma. They take this measurement, along with visual field tests and advanced imaging including optical coherence tomography (OCT) —which obtains high-resolution close-up photos of the retina—and use their best clinical judgment to decide how to care for a patient. Depending on these tests, patients are asked to come back for disease monitoring or start a treatment program.
What if there was a way to use these test results already collected from a patient to come up with more precise screening and monitoring methods? That’s what Dr. Zebardast’s research aims to accomplish.
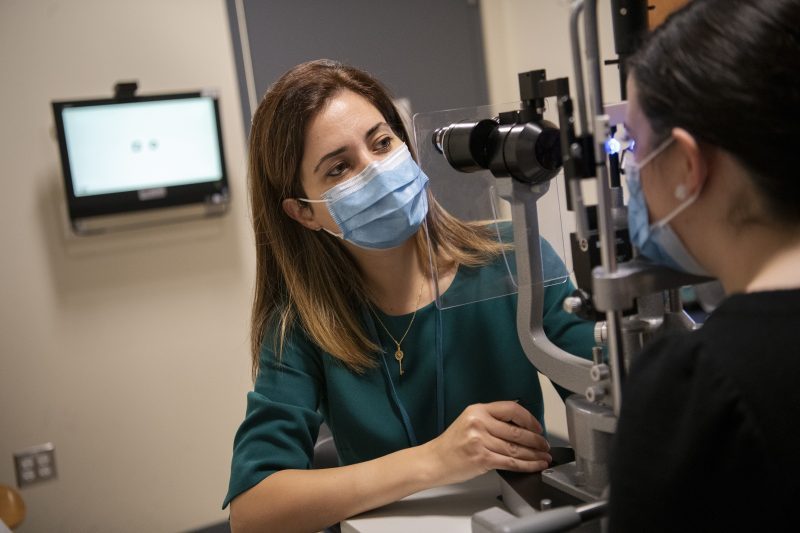
Machine learning to analyze imaging and the role of genetics
Experts have increasingly learned that glaucoma is a complex disease with various subtypes that are caused by various genes. A recent Mass Eye and Ear study identified at least 127 different genes associated with glaucoma. Researchers like Dr. Zebardast are looking at genetics for clues to glaucoma severity and finding ways to analyze a patient’s genetic risk. Additionally, they are investigating the role genetics plays in how successful glaucoma treatment will be. However, genetics is only one piece of the puzzle. Another important factor may be found in patients’ imaging.
Mass Eye and Ear has collected nearly 200,000 OCT images over time, while other research groups including the U.K.-based UK Biobank and NIH funded Ocular Hypertension Treatment Study (OHTS) also have each amassed a large bank of more than 100,000 images that can be harnessed for analysis.
This is where machine learning, a type of AI, can offer great value. Using a customized machine learning model, Dr. Zebardast and her team can analyze these “big data” sets comprised of hundreds of thousands of OCT images to look for structural similarities. These structures can represent new biomarkers for glaucoma that can help determine a patient’s disease course.
From there, the researchers can take a patient’s genetic information, gleaned from an affordable genetic test, and combine that with their imaging to assign a patient a risk score for glaucoma severity and likelihood of progression. Based on that score, a doctor can decide how often to have a patient come in for monitoring or how aggressively to treat the glaucoma. Conversely, a personalized risk score could save many patients with glaucoma from unnecessary treatment if their disease is unlikely to progress. Dr. Zebardast is hopeful that with further study, a basic model that can give patients a personalized risk score based on their genetics, visual field test, and OCT scans can be a reality within the next five years.
“This has never been done before, and can be applied to other diseases, too, by combining multiple pieces of information we obtain to come up with a risk score,” said Dr. Zebardast. “That’s the power of big data—we have all this information we collect as part of routine clinical care, and we need to harness that power to better understand individual disease.”
With support from a new four-year $300,000 grant from Research to Prevent Blindness, Dr. Zebardast will continue this work in the hopes of building prediction models that could forecast glaucoma progression.
“Dr. Zebardast is working at the cutting-edge of personalized medicine,” said David S. Friedman, MD, PhD, MPH, director of the Glaucoma Service at Mass Eye and Ear. “By identifying genes that are predictive of glaucoma and how those genes predict how severe glaucoma will be for an individual, and by using genetic information to identify response to therapy, Dr. Zebardast’s work will lead to improved identification of glaucoma patients and better care once they are in the clinic.”
From engineer to epidemiologist

Dr. Zebardast studied engineering physics as an undergraduate, where she was no stranger to math, computer science, and physics, before she pivoted to medicine. When she went to medical school, she was attracted to ophthalmology because of its use of advanced technology and physics. During her training, she got involved in epidemiology, which is how she first worked with data science. There, it became a natural progression to analyze big data and available health information, which she saw was being underutilized.
Glaucoma in particular piqued her interest because it can be an especially devastating disease for patients. Dr. Zebardast is also passionate about health equity, and is currently working on another big data project to better understand disparities in glaucoma care among different racial and ethnic groups, by examining large numbers of their medical records with advanced statistical techniques.
“One of the things that led me to glaucoma is that it’s a terrible blinding disease, the number one cause of irreversible blindness worldwide, and I wanted to make a difference there,” she said. “While there have been tremendous recent advances in glaucoma care, such as the introduction of minimally invasive glaucoma surgery, there is so much information lacking for the care we provide patients. More work needs to be done to better understand the disease and come up with more personalized ways to treat glaucoma.”


Interesting article…………….I know I am in the BEST hands with Dr. Zeebardast.
Thanks for your reading and your comment, Lois!
So, is current methodology: measuring pressure inside the eye, visual field tests, optical coherence tomography (OCT) to obtain high-resolution close-up photos of the retina, and doctor’s best clinical judgment–are these current tests and method, sufficient to diagnose glaucoma patients? Thanks!
I would like to thank you for what you are doing, growing up I have know of Glaucoma because my Grandmother had it. I never thought I would need surgery to keep what vision I have.
Thank you
Michael