The number 2020 holds special significance for eye care providers and vision scientists—it’s a reminder that in an ideal world, everyone everywhere would have 20/20 vision. As part of Harvard Ophthalmology’s 2020 Vision—A World Without Blindness initiative, Focus is highlighting some of the Department of Ophthalmology at Mass Eye and Ear’s biggest clinical achievements and breakthroughs.
Mass Eye and Ear and its Harvard Ophthalmology faculty are dedicated to providing exceptional clinical care with enhanced patient access to world-class treatment and the latest technology. Advances made in the past 20 years are shaping the future of eye care by integrating the latest scientific findings and individualized treatments based on a patient’s genetic makeup, disease risk factors and ability to access care.
Highlighted below are just a handful of groundbreaking clinical advances that have had a direct and meaningful impact on patient care at Mass Eye and Ear and have brought hope and healing to millions of people.
Retina
- Mass Eye and Ear and Harvard Ophthalmology clinician scientists were instrumental in the development of verteporfin photodynamic therapy (Visudyne®), the first FDA-approved treatment for wet age-related macular degeneration (AMD). Translational research breakthroughs from researchers also led to a new class of ophthalmic anti-vascular endothelial growth factor (VEGF) drugs, which have revolutionized patient care for AMD, diabetic macular edema and macular edema.
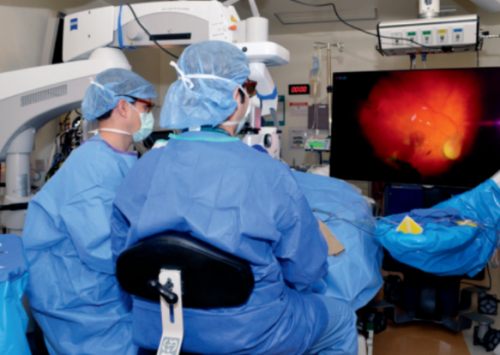
- In 2017, Mass Eye and Ear enhanced care for adult and pediatric retina patients by becoming the first hospital in New England to use a new and innovative vitreoretinal surgical platform, NGENUITY 3D Visualization System. Designed to enhance the operating experience for retina surgeons and their patients, the technology offers surgeons better visualization during surgery, fosters a more collaborative operating room environment and helps reduce surgeon fatigue.
Glaucoma
- Working with international collaborators, Mass Eye and Ear and Harvard Ophthalmology clinician-scientists have identified over 120 novel genetic risk factors for various forms of glaucoma. This work has provided critical new insights regarding the biology of the disease and may ultimately lead to new treatment targets and screening tools.
- Over the last two decades, glaucoma specialists at Mass Eye and Ear have been at the forefront of the latest minimally invasive glaucoma surgeries (MIGS) for patients of all ages. MIGS are designed to lower intraocular pressure, and these procedures often involve shorter recovery times and fewer risks than standard glaucoma operations.
Pediatric Ophthalmology
- In 2009, Mass Eye and Ear and Boston Children’s Hospital integrated their pediatric services to offer general pediatric ophthalmology and highly specialized pediatric strabismus care at both institutions. Collaborating to advance diagnostic and therapeutic approaches for childhood eye disorders enables clinicians to provide the most comprehensive pediatric ophthalmology network in the country.
- The Pediatric Ophthalmology and Strabismus Service offers advanced diagnostic services, including a saccadic vector optokinetic perimeter device to evaluate visual field changes in young patients—the first use of this technology in the United States. Other new capabilities include Optus imaging and the world’s only pediatric Adaptive Optics retinal imaging unit.
Cornea
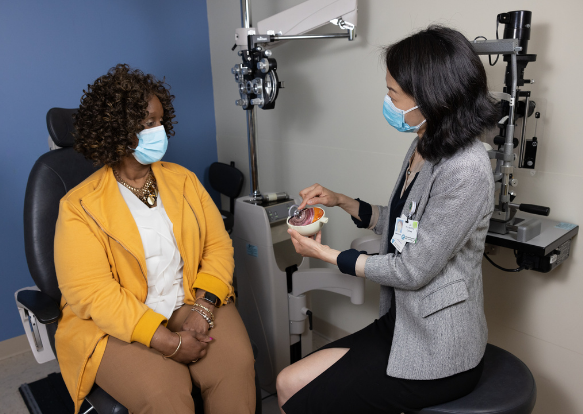
- In 2020, surgeons at Mass Eye and Ear were the first in the U.S. to replace the eye surface of patients who each experienced chemical burns to one eye—by using their own stem cells taken from the other healthy eye—in a technique known as cultivated autologous limbal epithelial cell transplantation (CALEC). This novel technique was developed by researchers at Mass Eye and Ear, Dana-Farber Cancer Institute and Boston Children’s Hospital.
- A leader in cornea and refractive surgery, Mass Eye and Ear was one of the first hospitals in New England to offer laser-assisted cataract surgery with the femtosecond LensSx® Laser.
- Mass Eye and Ear clinicians also provide the latest keratoplasty procedures for the treatment of Fuchs’ dystrophy, performing the first Descemet membrane endothelial keratoplasty in 2014 to treat the blinding eye disease.
Gene-Based Medicine
- In March 2018, Mass Eye and Ear made medical history by performing the first FDA-approved gene therapy procedure for patients with inherited blindness. The treatment, known as Luxturna™, is the first and only FDA-approved gene therapy treatment for an inherited disease.
- In 2016, Mass Eye and Ear started to provide investigators around the world with the ability to in-license adeno-associated viral vectors (Anc-AAVs) for the clinical development and commercialization of novel gene therapies. Anc-AAVs—first developed in the Grousbeck Gene Therapy Center at Mass Eye and Ear—have the potential to bring gene therapies to more patients than current generation viral vectors.
- In 2020, Mass Eye and Ear surgeons delivered an experimental CRISPR gene editing therapy inside the eye, the second case of its kind in the world, to treat a form of inherited blindness. This study is ongoing.
Ocular Imaging
- First introduced in 1991, optical coherence tomography (OCT) marked one of the greatest advances in ophthalmic imaging, as it plays an important role in the early detection of eye disease in patients to prevent vision loss. Researchers at Harvard Ophthalmology developed the technology in collaboration with colleagues at Massachusetts Institute of Technology and Massachusetts General Hospital.
- Today, researchers are studying the latest imaging techniques in the hopes of providing earlier diagnosis and treatment for patients with blinding eye diseases, such as AMD, glaucoma and diabetic retinopathy.
Telemedicine

- In response to COVID-19, Mass Eye and Ear quickly implemented virtual eye care visits, as well as hybrid visits that combine remote imaging with video or telephone conferencing, which continue to be in use today. The ability to monitor and manage disease via remote imaging provides a new level of convenience for patients and reduces the likelihood of cancelled appointments and delayed treatment.
Quality and Outcomes
- Since 2010, Mass Eye and Ear has led the medical community in the development and measurement of quality and outcomes in the field of ophthalmology. Nationwide, there is a lack of public-facing quality and outcomes reporting in the field of ophthalmology. Realizing the need for objective reporting, Mass Eye and Ear established its Quality Program—an institutional initiative that focuses on outcomes, provider excellence, clinical incidents response, and process improvement. Today, Mass Eye and Ear publishes one of the most comprehensive outcomes reports in the world for every ophthalmic subspecialty.
Clinical Data Science Institute
- Exciting advances in artificial intelligence and big data have the potential to transform patient care, and Harvard Ophthalmology is leading the way. In 2020, Harvard Ophthalmology established the Clinical Data Science Institute, which leverages big data to build stronger health profiles and predictive models so that we can improve the diagnosis and treatment of eye diseases.
Read More
 These are just some of the major advances that are highlighted in the most recent issue of Eye Witness. Read the full issue to discover how Harvard Ophthalmology clinician-scientists are planning to continue leading the way over the next 20 years.
These are just some of the major advances that are highlighted in the most recent issue of Eye Witness. Read the full issue to discover how Harvard Ophthalmology clinician-scientists are planning to continue leading the way over the next 20 years.



I’m 74 and was diagnosed with dry macular 10 years ago. Does not run in family. Is there medication to improve? I’m still able to see with strong glasses but wondering what research has found for DRY macular. Thank you in advance